EzriCare and Delsam Artificial Tears Recall – Everything You Need to Know
EzriCare and Delsam Artificial Tears Recall
In February 2023, Global Pharma Healthcare issued a recall for EzriCare Artificial Tears, Delsam Pharma Artificial Tears, and Delsam Pharma Artificial Eye Ointment due to a suspected bacterial contamination. According to the CDC, the contaminated eye products have resulted in vision loss, serious infections, and even death in some cases.
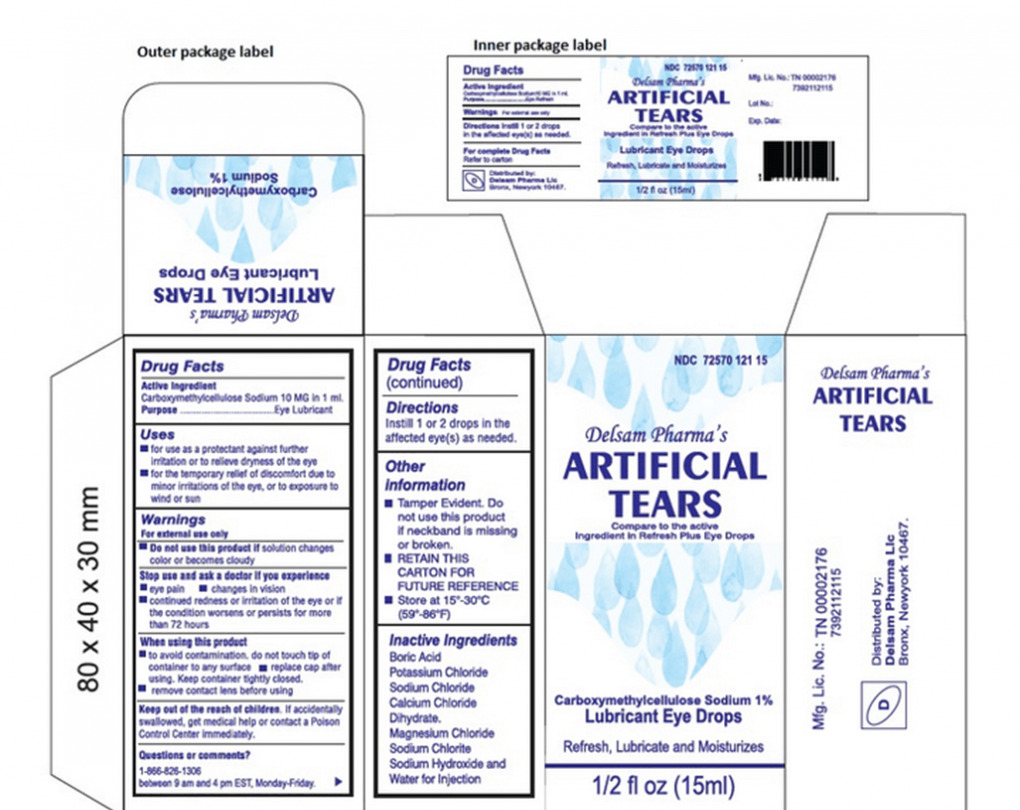
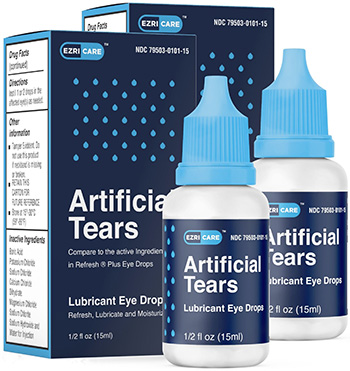
Which EzriCare and Delsam Eye Drops Were Recalled?
EzriCare and Delsam Artificial Tears were sold primarily through online platforms like Amazon and Walmart. CBS News reported that EzriCare Artificial Tears was among the top 10 products sold on Amazon for dry eye relief. Specific batches of these eye drops, including EzriCare Artificial Tears Lubricant Eye Drops and Delsam Pharma Artificial Tears and Lubricant Eye Drops, along with Delsam Pharma Artificial Eye Ointment were part of the recall.
Why Were EzriCare Artificial Tears Recalled?
These eye drops were recalled after the FDA and CDC linked the products to a multistate outbreak of a rare, drug-resistant strain of Pseudomonas aeruginosa bacterium known as Verona Integron-mediated Metallo-β-lactamase (VIM) and Guiana-Extended Spectrum-β-Lactamase (GES)-producing carbapenem-resistant Pseudomonas aeruginosa (VIM-GES-CRPA).
Risks of EzriCare Artificial Tears
The predominant risk of using EzriCare Artificial Tears is becoming infected with VIM-GES-CRPA. This strain of bacteria can cause severe eye infections leading to vision loss and has even resulted in three deaths, according to the CDC’s update as of March 21, 2023. Other side effects reported by users include eye pain, blurry vision, and allergic reactions.
FDA Response to Artificial Tears Recall
The FDA, in conjunction with the CDC, is conducting tests on unopened bottles of EzriCare eye drops to ascertain if the products were contaminated during the manufacturing process. As of the latest update on March 21, 2023, the results have not been released. Global Pharma has been placed on import alert by the FDA due to its failure to comply with current good marketing practices, preventing the company’s products from entering the U.S.
What Should I Do If I Used Recalled EzriCare Artificial Tears?
If you’ve used the recalled EzriCare Artificial Tears, it’s important to stop using the product immediately. If you experience any symptoms of infection, such as blurry vision, eye discharge, eye redness, eye pain, or increased light sensitivity, contact your healthcare provider as soon as possible.
If you believe you suffered an eye infection or vision loss after using EzriCare or Delsam Artificial Tears, don’t hesitate to contact our legal team for a free case review.
It is crucial to remain aware of product recalls and safety announcements to safeguard your health.